A) Z = 2A
B) Z = A
C) Z = A/2
D) ![]()
E) Z = A2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The 66Cu (Z = 29) produced in a nuclear bombardment is unstable, changing to 66Zn (Z = 30) by the emission of:
A) a proton
B) a gamma ray photon
C) a positron
D) an electron
E) an alpha particle
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of a radioactive isotope is 6.5 h. If there are initially 48 * 1032 atoms of this isotope, the number of atoms of this isotope remaining after 26 h is:
A) 12 * 1032
B) 6 * 1032
C) 3 * 1032
D) 6 * 104
E) 3 * 102
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The energies of electrons emitted in - decays have a continuous spectrum because:
A) the original neutron has a continuous spectrum
B) the neutrino can carry off energy
C) the emitted electron is free
D) energy is not conserved
E) the daughter nucleus may have any energy
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom of 235U (Z = 92) disintegrates to 207Pb (Z = 82) with a half-life of about a billion years by emitting seven alpha particles and ______ - particles:
A) 3
B) 4
C) 5
D) 6
E) 7
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass density of an atomic nucleus is:
A) about 1015 kg/m3
B) about 1012 kg/m3
C) increases with increasing nuclear mass
D) increases with decreasing nuclear radius
E) about the same as that of all other nuclei
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following collections of particles according to the total binding energy of all the particles in each collection, least to greatest. 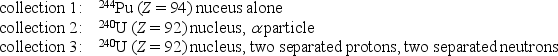
A) 1,2,3
B) 3,2,1
C) 2,1,3
D) 1,3,2
E) 2,3,1
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The becquerel is the correct unit to use in reporting the measurement of:
A) the rate of decay of a radioactive source
B) the ability of a beam of gamma ray photons to produce ions in a target
C) the energy delivered by radiation to a target
D) the biological effect of radiation
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an alpha decay the disintegration energy appears as:
A) photon energies
B) the kinetic energies of the alpha and the daughter nucleus
C) the excitation energy of the daughter nucleus
D) the excitation energy of the alpha particle
E) heat
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Volumes of atomic nuclei are proportional to:
A) the mass number
B) the atomic number
C) the total nuclear spin
D) the number of neutrons
E) none of these
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Some alpha emitters have longer half-lives than others because:
A) their alpha particles have greater mass
B) their alpha particles have less mass
C) their barriers to decay are higher and wider
D) their barriers to decay are lower and narrower
E) their decays include the emission of a photon
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following which has the smallest rest energy?
A) A neutron
B) An electron
C) An ion
D) A proton
E) An atom
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A beta particle is:
A) a helium nucleus
B) an electron or a positron
C) a radioactive element
D) any negative particle
E) a hydrogen atom
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a nucleus has mass M, Z protons (mass mp) and N neutrons (mass mn) its binding energy is equal to:
A) Mc2
B) (M - Zmp - Nmn) c2
C) (Zmp + Nmn - M) c2
D) (Zmp + Nmn) c2
E) (Zmp - M) c2
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A nucleus with mass number A and atomic number Z emits an alpha particle. The mass number and atomic number, respectively, of the daughter nucleus are:
A) A, Z -2
B) A - 2, Z - 2
C) A - 2, Z
D) A - 4, Z
E) A - 4, Z - 2
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Let Z denote the atomic number and A denote the mass number of a nucleus. The number of neutrons in this nucleus is:
A) Z
B) A - Z
C) A - 2Z
D) A
E) 2A - Z
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Starting with a sample of pure 66Cu, 7/8 of it decays into Zn in 15 minutes. The corresponding half-life is:
A) 15 minutes
B) 5 minutes
C) 7 minutes
D) 3.75 minutes
E) 10 minutes
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Iron has atomic number 26. Naturally mined iron contains isotopes of mass numbers 54, 56, 57, and 58. Which of the following statements is FALSE?
A) every atom of iron has 26 protons
B) some iron atoms have 30 neutrons
C) some iron atoms have 54 neutrons
D) the isotopes may be separated in a mass spectrometer
E) there are four kinds of naturally occurring iron atoms with the same chemical properties
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A radioactive atom X emits a - particle. The resulting atom:
A) must be very reactive chemically
B) has an atomic number that is one more than that of X
C) has a mass number that is one less than that of X
D) must be radioactive
E) is the same chemical element as X
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The relation between the disintegration constant and the half-life T of a radioactive substance is:
A) = 2T
B) = 1/T
C) = 2/T
D) T = ln 2
E) T = ln(1/2)
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 67
Related Exams