Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sodium carbonate, Na2CO3(s) , can be prepared by heating sodium bicarbonate, NaHCO3(s) . 2NaHCO3(s)  Na2CO3(s) + CO2(g) + H2O(g) Kp = 0.23 at 100ºC
If a sample of NaHCO3 is placed in an evacuated flask and allowed to achieve equilibrium at 100ºC, what will the total gas pressure be?
Na2CO3(s) + CO2(g) + H2O(g) Kp = 0.23 at 100ºC
If a sample of NaHCO3 is placed in an evacuated flask and allowed to achieve equilibrium at 100ºC, what will the total gas pressure be?
A) 0.46 atm
B) 0.96 atm
C) 0.23 atm
D) 0.48 atm
E) 0.11 atm
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Ethanol and acetic acid react to form ethyl acetate and water according to the following chemical equation:
C2H5OH(l)+ CH3COOH(l) ![Ethanol and acetic acid react to form ethyl acetate and water according to the following chemical equation: C<sub>2</sub>H<sub>5</sub>OH(l)+ CH<sub>3</sub>COOH(l) CH<sub>3</sub>COOC<sub>2</sub>H<sub>5</sub>(l)+ H<sub>2</sub>O(l) When two moles each of ethanol and acetic acid are combined, equilibrium is reached when two-thirds of a mole of each of the reactants remains. Calculate the equilibrium constant for this reaction assuming total volume does not change during the course of the reaction. (Note: since water is not the solvent, but rather a product of the reaction, [H<sub>2</sub>O] changes during the course of the reaction, so it should be included in the equilibrium constant expression.)](https://d2lvgg3v3hfg70.cloudfront.net/TB3244/11ea7995_6663_6851_911b_1f4b8d143e02_TB3244_00.jpg) CH3COOC2H5(l)+ H2O(l)
When two moles each of ethanol and acetic acid are combined, equilibrium is reached when two-thirds of a mole of each of the reactants remains. Calculate the equilibrium constant for this reaction assuming total volume does not change during the course of the reaction. (Note: since water is not the solvent, but rather a product of the reaction, [H2O] changes during the course of the reaction, so it should be included in the equilibrium constant expression.)
CH3COOC2H5(l)+ H2O(l)
When two moles each of ethanol and acetic acid are combined, equilibrium is reached when two-thirds of a mole of each of the reactants remains. Calculate the equilibrium constant for this reaction assuming total volume does not change during the course of the reaction. (Note: since water is not the solvent, but rather a product of the reaction, [H2O] changes during the course of the reaction, so it should be included in the equilibrium constant expression.)
Correct Answer

verified
Correct Answer
verified
Short Answer
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide.
CaCO3(s) 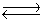 CaO(s)+ CO2(g)
KP for this reaction is 1.16 at 800°C. A 5.00 L vessel containing 10.0 g of CaCO3(s)was evacuated to remove the air, sealed, and then heated to 800°C. Ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
CaO(s)+ CO2(g)
KP for this reaction is 1.16 at 800°C. A 5.00 L vessel containing 10.0 g of CaCO3(s)was evacuated to remove the air, sealed, and then heated to 800°C. Ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the reaction 2H2S(g)  2H2(g) + S2(g) is carried out at 1065°C, Kp = 0.012. Starting with pure H2S at 1065°, what must the initial pressure of H2S be if the equilibrated mixture at this temperature is to contain 0.250 atm of H2(g) ?
2H2(g) + S2(g) is carried out at 1065°C, Kp = 0.012. Starting with pure H2S at 1065°, what must the initial pressure of H2S be if the equilibrated mixture at this temperature is to contain 0.250 atm of H2(g) ?
A) 1.06 atm
B) 1.86 atm
C) 0.94 atm
D) 0.90 atm
E) 1.52 atm
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
The data below refer to the following reaction:
2NO(g)+ Br2(g) 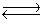 2NOBr(g)
2NOBr(g)  Find the concentration of NOBr when the system reaches equilibrium.
Find the concentration of NOBr when the system reaches equilibrium.
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the following equilibrium,
4NH3(g)+ 3O2(g) 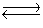 2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the reactants would increase, decrease, or remain constant after ammonia was added to the system.
2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the reactants would increase, decrease, or remain constant after ammonia was added to the system.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 700 K, the reaction 2SO2(g) + O2(g) ![At 700 K, the reaction 2SO<sub>2</sub>(g) + O<sub>2</sub>(g) 2SO<sub>3</sub>(g) has the equilibrium constant K<sub>c</sub> = 4.3 * 10<sup>6</sup> , and the following concentrations are present: [SO<sub>2</sub>] = 0.10 M; [SO<sub>3</sub>] = 10. M; [O<sub>2</sub>] = 0.10 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written) , left to right or right to left, will the reaction proceed to reach equilibrium? A) Yes, the mixture is at equilibrium. B) No, left to right C) No, right to left D) There is not enough information to be able to predict the direction.](https://d2lvgg3v3hfg70.cloudfront.net/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) 2SO3(g) has the equilibrium constant Kc = 4.3 * 106 , and the following concentrations are present: [SO2] = 0.10 M; [SO3] = 10. M; [O2] = 0.10 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written) , left to right or right to left, will the reaction proceed to reach equilibrium?
2SO3(g) has the equilibrium constant Kc = 4.3 * 106 , and the following concentrations are present: [SO2] = 0.10 M; [SO3] = 10. M; [O2] = 0.10 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written) , left to right or right to left, will the reaction proceed to reach equilibrium?
A) Yes, the mixture is at equilibrium.
B) No, left to right
C) No, right to left
D) There is not enough information to be able to predict the direction.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction A(g) + 2B(g)  C(g) was allowed to come to equilibrium. The initial amounts of reactants placed into a 5.00 L vessel were 1.0 mol A and 1.8 mol B. After the reaction reached equilibrium, 1.0 mol of B was found. Calculate Kc for this reaction.
C(g) was allowed to come to equilibrium. The initial amounts of reactants placed into a 5.00 L vessel were 1.0 mol A and 1.8 mol B. After the reaction reached equilibrium, 1.0 mol of B was found. Calculate Kc for this reaction.
A) 0.060
B) 5.1
C) 17
D) 19
E) 25
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Kp for the reaction of SO2(g) with O2 to produce SO3(g) is 3 * 1024 . Calculate Kc for this equilibrium at 25ºC. (The relevant reaction is 2SO2(g) + O2(g)  2SO3(g) .)
2SO3(g) .)
A) 3 * 1024
B) 5 * 1021
C) 2 * 1020
D) 5 * 1022
E) 7 * 1025
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the nitrogen fixation reaction 3H2(g) + N2(g)  2NH3(g) , Kc = 6.0 * 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?
2NH3(g) , Kc = 6.0 * 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?
A) 0.750 M
B) 2.7 M
C) 0.250 M
D) 0.025 M
E) 1.85 M
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reactions and their associated equilibrium constants: A + 2B  C K1
C 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 D + E K2
For the reaction A + 2B D + E, having equilibrium constant Kc,
C K1
C 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 D + E K2
For the reaction A + 2B D + E, having equilibrium constant Kc,
A) Kc = K1 + K2
B) Kc = K1/K2
C) Kc = K1 - K2
D) Kc = (K1) (K2)
E) Kc = K2/K1
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The equilibrium constant for the chemical equation
2NO(g)+ O2(g) 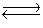 2NO2(g)
is two times the equilibrium constant for the chemical equation
NO(g)+ 1/2O2(g)
2NO2(g)
is two times the equilibrium constant for the chemical equation
NO(g)+ 1/2O2(g) 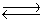 NO2(g).
NO2(g).
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the following equilibrium,
4NH3(g)+ 3O2(g) 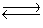 2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the products would increase, decrease, or remain constant after ammonia was added to the system.
2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the products would increase, decrease, or remain constant after ammonia was added to the system.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following equilibria: 2SO3(g)  2SO2(g) + O2(g) Kc = 2.3 * 10-7
2NO3(g) 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 2NO2(g) + O2(g) Kc = 1.4 * 10-3
Calculate the equilibrium constant for the reaction
SO2(g) + NO3(g) SO3(g) + NO2(g)
2SO2(g) + O2(g) Kc = 2.3 * 10-7
2NO3(g) 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 2NO2(g) + O2(g) Kc = 1.4 * 10-3
Calculate the equilibrium constant for the reaction
SO2(g) + NO3(g) SO3(g) + NO2(g)
A) 78
B) 1.3 * 10-2
C) 1.6 * 10-4
D) 3.2 * 10-10
E) 6.1 * 103
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If one starts with pure NO2(g) at a pressure of 0.500 atm, the total pressure inside the reaction vessel when 2NO2(g)  2NO(g) + O2(g) reaches equilibrium is 0.674 atm. Calculate the equilibrium partial pressure of NO2.
2NO(g) + O2(g) reaches equilibrium is 0.674 atm. Calculate the equilibrium partial pressure of NO2.
A) 0.152 atm
B) 0.174 atm
C) 0.200 atm
D) 0.326 atm
E) The total pressure cannot be calculated because Kp is not given
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
3. 2NO2 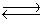 2NO + O2 Kp = 5.9 *10 -5
2NO + O2 Kp = 5.9 *10 -5
A) 2 < 1 < 3
B) 1 < 2 < 3
C) 2 < 3 < 1
D) 3 < 2 < 1
E) 3 < 1 < 2
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 250ºC, the equilibrium constant Kp for the reaction PCl5(g)  PCl3(g) + Cl2(g) is 1.80. Sufficient PCl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250ºC. Calculate the pressure of PCl5 after the system has reached equilibrium.
PCl3(g) + Cl2(g) is 1.80. Sufficient PCl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250ºC. Calculate the pressure of PCl5 after the system has reached equilibrium.
A) 1.50 atm
B) 1.24 atm
C) 4.24 atm
D) 0.94 atm
E) 1.12 atm
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the following equilibrium,
4NH3(g)+ 3O2(g) 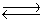 2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the reactants would increase, decrease, or remain constant when the temperature is increased.
2N2(g)+ 6H2O(g)+ 1531 kJ
State whether the concentrations of the reactants would increase, decrease, or remain constant when the temperature is increased.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the common allotropes of carbon (graphite and diamond) , C(gr) C(dia) with equilibrium constant K = 0.32. The molar volumes of graphite and diamond are, respectively, 5.30 cm3/mol and 3.42 cm3/mol; Hf of diamond is 1.90 kJ/mol. This data suggests that the formation of diamond is favored at
A) low temperatures and low pressures.
B) high temperatures and low pressures.
C) low temperatures and high pressures.
D) high temperatures and high pressures.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 99
Related Exams