A) antimycin
B) neomycin
C) oligomycin
D) penicillin
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the enzyme catalyzed reaction sequence below,can the E-PO₄- intermediate be predicted and why?
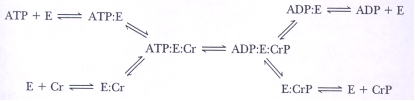
A) Yes,the mechanism is a double-displacement reaction.
B) Yes,the reaction fits the Ping-Pong model.
C) No,the reaction is random single displacement.
D) No,the reaction is double displacement.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A plot of 1/V versus 1/[S] for an enzyme-catalyzed reaction gave a line with an equation of y = 0.5x + 0.2.The same enzyme with an inhibitor present gave a line with an equation of y = 1.1x + 0.2.Which of these statements is correct regarding the inhibition?
A) It is competitive.
B) It is non-competitive.
C) It is uncompetitive.
D) It is irreversible.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is correct regarding the Michaelis-Menten constant,Km,for this kinetic mechanism?
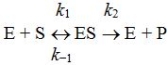
A) Km is numerically equal to the substrate concentration required to achieve one half the maximum velocity.
B) Its defined as Km = k1/(k-1 + k2) .
C) Km is approximately equal to the dissociation constant for the enzyme-substrate complex to E + P.
D) The numeric value of Km has the units of mole-1.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a feature of uncompetitive inhibition?
A) I combines only with ES.
B) I combines only with E.
C) I combines with E and ES.
D) I combines with EP.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the basis for the International Units of an enzyme?
A) ratio of enzyme to other proteins
B) micromoles of product formed per minute
C) moles of substrate reacted
D) micromoles of product produced at Vmax/2
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What term describes an enzyme that is an ATP-dependent phosphotransferase?
A) isomerase
B) uricase
C) protease
D) kinase
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which reaction would NOT proceed via bimolecular elementary steps?
A) C + D→ T + U
B) a reaction with a rate constant in the units of s-1
C) 2A →D + E
D) a reaction with a molecularity of 2
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which term defines the catalytically active complex of an apoenzyme and its prosthetic group?
A) catalytic duo
B) holoenzyme
C) prosthetic enzyme
D) dimeric enzyme
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For an enzyme-catalyzed reaction,the initial velocity was determined at 2 different concentrations of the substrate.Which of the following would be closest to the value of Vmₐₓ?
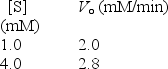
A) 0.32 mM/min
B) 0.67 mM/min
C) 1.5 mM/min
D) 3.19 mM/min
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the reaction mechanism below,which components are competitive for binding to free enzyme E?
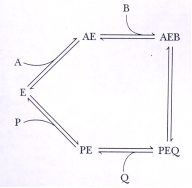
A) P and A
B) A and B
C) B and Q
D) Q and A
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the definition of the free energy of activation,∆G‡?
A) the average free energy of the product formed
B) the rate of a chemical reaction in relationship to the concentration of reactant molecules
C) the energy required to raise the average energy of one mole of reactant to the transition state energy
D) the amount of energy released by a spontaneous reaction
F) C) and D)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following is NOT correct regarding enzyme pathways?
A) The most effective way to control the pathway is to regulate every enzyme in the pathway.
B) An enzyme pathway always proceeds in only one direction,never in reverse.
C) A regulatory enzyme is regulated only by molecules within the given pathway.
D) Metabolic pathways are necessary since enzymes usually catalyze only one specific reaction.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT correct regarding non-competitive inhibition?
A) Non-competitive inhibitors interact with the enzyme as well as the enzyme-substrate complex.
B) Increasing the concentration of [S] can overcome the inhibition.
C) The Vmax value does not remain the same as for a reaction that is not inhibited.
D) The inhibitor can cause a conformational change in the enzyme.
F) A) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Against which type of antigen is a catalytic antibody (abzyme) generated?
A) an analog of the transition-state intermediate in the reaction
B) the substrate of the reaction
C) an analog of the product of the reaction
D) an analog of the transition-state intermediate in the reaction.
F) A) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
If an enzyme has a Vmₐₓ of 15 mM/min,what is the velocity if the substrate is present at ¼ of the Km?
A) 12 mM/min
B) 6 mM/min
C) 3.75 mM/min
D) 3 mM/min
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a distinctive feature of enzymes?
A) regulatory
B) catalytic activity
C) ability to change ∆G
D) specificity
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT correct regarding the transition state of a reaction?
A) The transition state is located at the height of a free energy diagram.
B) The transition state,once reached,indicates that there is a high probability that the reaction will occur.
C) The energy required to raise the average energy of one mole of reactant to the transition state is the free energy of activation.
D) The transition state energy level is the sum of the energy levels of the reactants and products.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT correct for catalysts?
A) They work by lowering the energy of activation.
B) The average energy of the reaction is unchanged.
C) They combine transiently with the reactants,promoting a reactive transition state condition.
D) They are regenerated after each third reaction cycle.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a characteristic of ribozymes?
A) They emerge from the reaction changed.
B) They are substrate specific.
C) They enhance the reaction rate.
D) They are RNA molecules.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 43
Related Exams