A) II, III
B) I, III, IV
C) III, IV
D) I, II, III
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Identify the acid, base, conjugate acid, conjugate base in the following reaction.
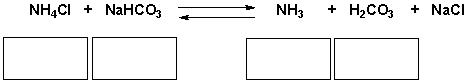
Correct Answer

verified
acid, base...View Answer
Show Answer
Correct Answer
verified
View Answer
True/False
Lewis bases donate electrons when reacting.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The equilibrium constant will be greater than 1.0 for the following reaction
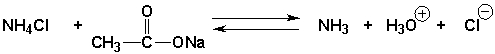
B) False
Correct Answer

verified
False
Correct Answer
verified
Multiple Choice
Which are acid-base reactions according to Brønsted-Lowry theory?
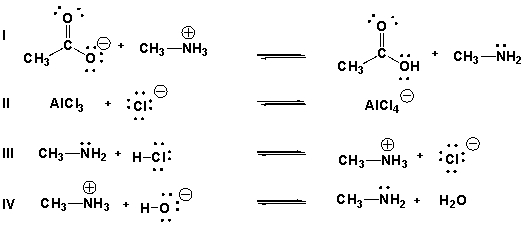
A) I, III
B) I, II, II, IV
C) I, II, III
D) I, III, IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the role of water in the following reaction?

A) acid
B) base
C) conjugate acid
D) conjugate base
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which equilibria have equilibrium constants smaller than 1.0?
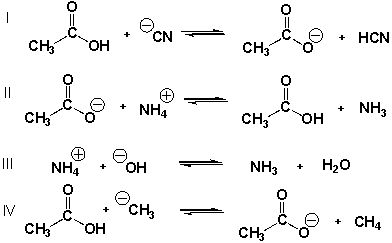
A) II
B) I, IV
C) III, IV
D) I
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the role of methanol (CH3OH) in the following reaction?
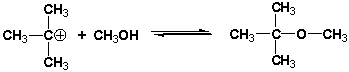
A) Lewis acid
B) Lewis base
C) Brønsted acid
D) Brønsted base
F) A) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of these has the lowest numerical value of pKa and is therefore the strongest acid?
A) CH3COOH
B) H2O
C) NH4+
D) HCl
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statements about acid-base equilibria are true? I. The pKa is the negative log10 of the acid equilibrium constant. II. A stronger acid has a pKa with a smaller value than a weaker acid. III. The stronger the base, the smaller the pKa of its conjugate acid. IV. The Ka = K [HA].
A) I, III
B) I, II
C) I, II, III
D) II, III, IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the stronger base if the equilibrium lies to the right? (Sec. 2.4, HARD)
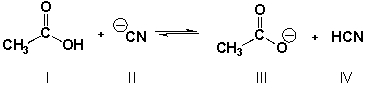
A) I
B) II
C) III
D) IV
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List the bonds in order of decreasing acidity (most to least) .

A) I, III, II, IV
B) IV, II, III, I
C) II, III, IV, I
D) I, II, III, IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The strongest acid in the following list is sodium bicarbonate.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following species in order of increasing basicity (weakest to strongest) . I. OH- II. Cl- III. H2O IV. NH3
A) II, III, IV, I
B) III, I, IV, II
C) IV, I, II, III
D) III, IV, I, II
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which are acid-base reactions according to Lewis theory but not according to the Brønsted-Lowry theory?
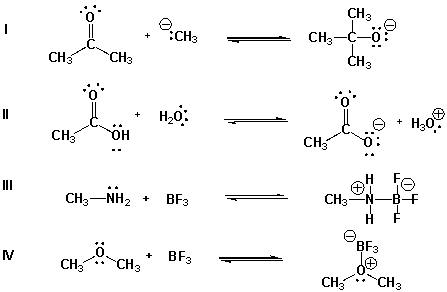
A) I, II
B) III, IV
C) I, III, IV
D) I, II, III, IV
F) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
Ammonia acts as a Brønsted-Lowry base in the following reaction.

B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the order of decreasing acid strength of the following compounds (greatest first) ?
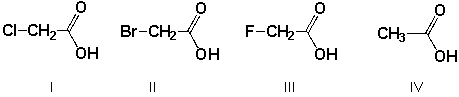
A) II, I, III, IV
B) III, IV, I, II
C) III, I, II, IV
D) IV, II, I, III
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the proper reaction mechanism for the reaction of boron trifluoride and diethyl ether?
Assume that the charges are correct and add electron pairs, if needed. Also consider how many electrons should be in the outer shell of boron trifluoride to make it neutral.
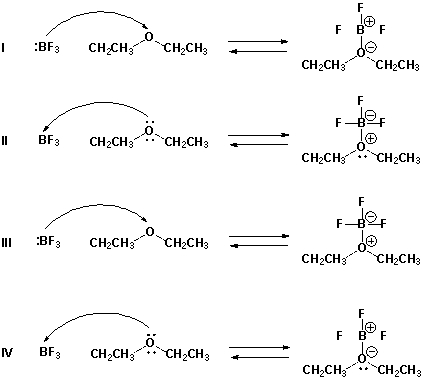
A) I
B) II
C) III
D) IV
F) A) and C)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Identify the Arrhenius bases: I. NH3 II. NaOH III. HI IV. Ca(OH) 2
A) I, II
B) II, IV
C) I, III
D) I, II, IV
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Complete the following reaction scheme with the appropriate equilibrium arrow (indicating the higher concentrations at equilibrium).

Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 47
Related Exams