A) nearby presence of photons of same wavelength as those emitted
B) high flux of electrons
C) high voltage
D) high temperature
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The wavelength of a coherent laser is 628 nm. What energy difference exists between the upper excited state involved and the lower unexcited ground state? (h = 6.63 *10-34 J.s, c = 3.00 *108 m/s, 1 eV = 1.60 *10-19 J, and 1 nm = 10-9 m)
A) 1.86 eV
B) 1.81 eV
C) 1.75 eV
D) 1.98 eV
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A photon is emitted from a hydrogen atom that undergoes a transition from n = 3 to n = 2. Calculate the energy and wavelength of the photon. (The ionization energy of hydrogen is 13.6 eV, and h = 6.63 *10-34 J.s, c = 3.00 * 108 m/s, 1 eV = 1.60 *10-19 J, and 1 nm = 10-9 m)
A) 2.21 eV, 563 nm
B) 1.89 eV, 460 nm
C) 2.58 * 10-19 J, 658 nm
D) 3.02 * 10-19 J, 658 nm
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a hydrogen atom absorbs a photon that raises it to the n = 5 state, how many different energies are possible for the photon(s) that may be emitted as the atom eventually returns to the ground state?
A) 5
B) 3
C) 4
D) The correct value is not given.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lyman series of hydrogen is made up of those transitions made from higher levels to n = 1. If the first line (n = 2 to n = 1) in this series has a wavelength of 122 nm, what is the wavelength of the third line (n = 4 to n = 1) ?
A) 364 nm
B) 97.6 nm
C) 103 nm
D) 486 nm
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The quantum mechanical model of the hydrogen atom requires that if the principal quantum number = 6, there will be how many permitted orbital quantum numbers?
A) 3
B) 16
C) 6
D) 5
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an analysis relating Bohr's theory to the de Broglie wavelength of electrons, when an electron moves from the n = 1 level to the n = 3 level, the circumference for its orbit becomes 9 times greater. This occurs because:
A) the wavelength of the electron becomes nine times as long.
B) there are triple as many wavelengths, and each wavelength is triple in length.
C) there are nine times as many wavelengths in the new orbit.
D) the electron is moving nine times as fast.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are in chlorine's (atomic number 17) next to outer shell (n = 2) ?
A) 18
B) 4
C) 2
D) 8
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum number of electrons that can occupy a 3d subshell?
A) 5
B) 14
C) 10
D) 18
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the wavelengths emitted from a hydrogen gas discharge tube, those that are associated with transitions from higher levels down to the n = 1 level produce which of the following?
A) infrared
B) visible
C) ultraviolet
D) mixture of infrared and visible
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Characteristic K X-rays are the result of:
A) inner electron transitions.
B) outer electron transitions.
C) nuclear electron states.
D) buckytubes.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Bohr theory does not predict that:
A) the approximate radius of a hydrogen atom is 5.3 *10-11 m.
B) hydrogen atoms will give off the lines from the series ending in the n = 2 state.
C) the ground state of hydrogen is spherically symmetric.
D) it requires 13.6 eV to ionize hydrogen.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The quantum mechanical model of the hydrogen atom requires that if the orbital quantum number = 5, there will be how many permitted orbital magnetic quantum numbers allowed?
A) 7
B) 15
C) 5
D) 11
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a wire carries high current causing it to glow, it will emit which type of spectrum?
A) line absorption
B) monochromatic
C) continuous
D) line emission
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The restriction that no more than one electron may occupy a given quantum state in an atom was first stated by which of the following scientists?
A) Bohr
B) Pauli
C) de Broglie
D) Heisenberg
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In contrast to the plum-pudding model of the atom, Rutherford's model:
A) was first to explain atoms emitting discrete frequencies.
B) had the positive charge concentrated in a small region.
C) eliminated radiation from accelerating charges.
D) had the positive charge spread uniformly through the atom.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Paschen series of hydrogen corresponds to electron transitions from higher levels to n = 3. What is the longest wavelength in that series? (h = 6.63 * 10-34 J.s. The ground state of hydrogen is at -13.6 eV.)
A) 822 nm
B) 365 nm
C) 1880 nm
D) 1094 nm
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following transitions in hydrogen from an initial state (ni) to a final state (nf) results in the least energy emitted?
A) ni = 3, nf = 95
B) ni = 80, nf = 2
C) ni = 2, nf = 1
D) ni = 1, nf = 3
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The quantum mechanical model of the hydrogen atom requires that if the principal quantum number = 3, there will be permitted how many orbital magnetic quantum numbers?
A) 5
B) 8
C) 4
D) 7
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the n = 5 shell, what are the lowest values possible for 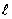 and
and 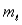 respectively?
respectively?
A) "-5, -5"
B) "-4, -4"
C) "0, -4"
D) "0, 0"
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 63
Related Exams